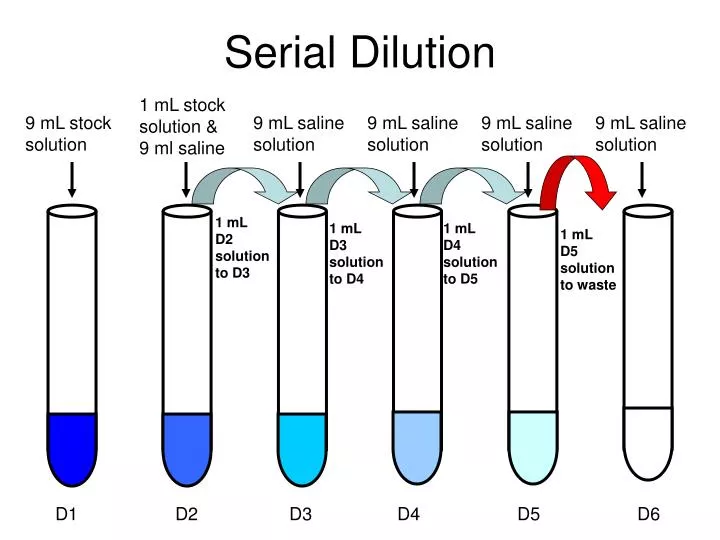
Dilution Preparation . dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated. If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). pipets and volumetric flasks are used when we need to know a solution’s exact concentration; often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. follow these five steps to make a dilution: This reference contains standard dilutions.
from dxorvxlft.blob.core.windows.net
often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. This reference contains standard dilutions. If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). Of course, the resulting solution is. dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. follow these five steps to make a dilution: pipets and volumetric flasks are used when we need to know a solution’s exact concentration; to dilute a solution means to add more solvent without the addition of more solute. dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated.
Serial Dilutions Lab at Joseph Brown blog
Dilution Preparation dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated. This reference contains standard dilutions. dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). Of course, the resulting solution is. dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated. often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. pipets and volumetric flasks are used when we need to know a solution’s exact concentration; follow these five steps to make a dilution: to dilute a solution means to add more solvent without the addition of more solute.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Preparation often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. follow these five steps to make a dilution: pipets and volumetric flasks are used when we need to know a solution’s exact concentration; Of course, the resulting solution is. This reference contains standard dilutions. If you. Dilution Preparation.
From onelab.andrewalliance.com
Simple Serial Dilution Preparation Protocol OneLab Dilution Preparation to dilute a solution means to add more solvent without the addition of more solute. often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. follow these five steps to make a dilution: Of course, the resulting solution is. dilution with a small volume of. Dilution Preparation.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog Dilution Preparation If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated. pipets and volumetric flasks are used when we need. Dilution Preparation.
From www.youtube.com
Préparation d'une solution Dissolution et dilution YouTube Dilution Preparation to dilute a solution means to add more solvent without the addition of more solute. dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. If. Dilution Preparation.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Preparation pipets and volumetric flasks are used when we need to know a solution’s exact concentration; This reference contains standard dilutions. dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is. Dilution Preparation.
From dxobyjbye.blob.core.windows.net
How To Make Dilutions In Lab at Drew Ballard blog Dilution Preparation follow these five steps to make a dilution: pipets and volumetric flasks are used when we need to know a solution’s exact concentration; If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). to dilute a. Dilution Preparation.
From www.youtube.com
150 dilution.why need this dilution?2 easy methods to preparation Dilution Preparation follow these five steps to make a dilution: If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock. Dilution Preparation.
From www.pinterest.com
How to Do Serial Dilutions 9 Steps (with Pictures) Science fair Dilution Preparation pipets and volumetric flasks are used when we need to know a solution’s exact concentration; to dilute a solution means to add more solvent without the addition of more solute. If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you. Dilution Preparation.
From www.youtube.com
How to Perform a Bacterial Dilution Calculation YouTube Dilution Preparation dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. follow these five steps to make a dilution: often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. to dilute a solution means to add. Dilution Preparation.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram Dilution Preparation pipets and volumetric flasks are used when we need to know a solution’s exact concentration; dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. This reference contains standard dilutions. dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is. Dilution Preparation.
From www.youtube.com
How to Make a Dilution of a Stock Solution YouTube Dilution Preparation dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. pipets and volumetric flasks are used when we need to know a solution’s exact concentration; to dilute a solution means to add more solvent without the addition of more solute. often, it is convenient to prepare. Dilution Preparation.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Preparation If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). to dilute a solution means to add more solvent without the addition of more solute. pipets and volumetric flasks are used when we need to know a. Dilution Preparation.
From exoaaybok.blob.core.windows.net
Dilution Method Microbiology at Harry Hausman blog Dilution Preparation often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. follow these five steps to make a dilution: dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated. Of course, the resulting solution is. to dilute a. Dilution Preparation.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Preparation often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. This reference contains standard dilutions. If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). follow. Dilution Preparation.
From www.schoolmouv.fr
Composition d'un mélange cours de Seconde Physiquechimie Dilution Preparation dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. If you do not know them already, use the dilution factor equation to calculate the volume of. Dilution Preparation.
From cewxohfx.blob.core.windows.net
Dilution And Solution Preparation at Rita Favela blog Dilution Preparation dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. This reference contains standard dilutions. follow these five steps to make a dilution: Of course, the resulting solution is. often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single. Dilution Preparation.
From cejakhtq.blob.core.windows.net
How To Do A Dilution Experiment at Lillian Benda blog Dilution Preparation If you do not know them already, use the dilution factor equation to calculate the volume of the original solution (v1) and the volume of the dilution you are making (v2). follow these five steps to make a dilution: Of course, the resulting solution is. dilution with a small volume of an appropriate vehicle and administration using a. Dilution Preparation.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog Dilution Preparation Of course, the resulting solution is. often, it is convenient to prepare a series of solutions of known concentrations by first preparing a single stock solution, as. dilution with a small volume of an appropriate vehicle and administration using a motorised infusion pump is advocated. This reference contains standard dilutions. to dilute a solution means to add. Dilution Preparation.